Abstract
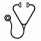
Background and study design. Reduced intensity conditioning transplantation (RICT) is a commonly applied treatment option for AML patients >50 years of age. Prospective, controlled studies comparing RICT with standard chemotherapy are warranted. In this study, we aimed to prevent selection biases. Thus, patients were included prior to HLA typing of potential sibling donors, and statistical analyses were based on an intention-to-transplant, donor versus no-donor approach. Hence, the analyses also include events occurring during the donor search period and also the transplantation procedures along with post-transplant events.
Patients and procedures. Between 2003 and 2016, 163 patients with AML in CR1 were included in Canada (n=69), Sweden (n=63), and Germany/Finland/Norway/New Zealand (n=31). Eighteen patients were excluded due to enrolment after the start of donor typing (n=14), lack of data (n=1), low-risk AML (n=2) or withdrawn consent (n=1). Thus, results from 145 patients with high (n=48) or intermediate (n=97) risk disease were available for analysis. Included patients were a median of 63 (50-70) years old, deemed fit for RICT and had at least one willing and healthy but not yet HLA typed sibling. The ensuing HLA typings thus yielded one RICT group including patients with ≥1 confirmed matched sibling donor (MSD), and one control group with no MSD. Date of inclusion was defined as date of HLA typing of the first potential MSD. The protocol-specified conditioning regimen for RICT was fludarabine (150-180 mg/m2) and busulfan (8 mg/kg orally or 6.4 mg/kg i.v., used in 95% of patients). Immunosuppression was ciclosporin alone (9%), with methotrexate (53%) or with MMF (35%). Peripheral blood stem cells were used in 95% of transplantations. Control patients received consolidation chemotherapy as per local routines.
Statistics. Baseline factors were compared between study groups using Fisher´s exact test and rank sum tests. The primary endpoint was overall survival (OS) with secondary endpoints of relapse-free survival (RFS), relapse incidence (RI) and non-relapse mortality (NRM). Kaplan-Meier curves were used to estimate OS and RFS and cumulative incidence functions were used to estimate NRM and RI considering competing risks. The logrank test was employed for group comparisons of event rates with time censored at 5 yrs post inclusion.
Results. The median follow-up time of surviving patients was 7.9 (0.24-14) yrs. Age, AML risk group and time from CR to inclusion did not differ between the study groups.
Time lag from diagnosis to study inclusion was 65 (32-256) days (Controls) and 64 (29-319) days (RICT), P=0.74, Mann-Whitney test. Time from CR1 to inclusion was 22 (0-218) days (Controls) and 19 (0-131) (RICT).
Excluding conditioning, patients in the RICT group received fewer chemotherapy cycles than controls. The time from start of last chemotherapy to transplant was median 63 (36-212) days. The incidence of acute (grade 2-4) and chronic extensive GvHD in transplanted patients was 25% and 39%, respectively.
The non-relapse mortality at 3 years post inclusion (Table) was 12% (RICT group) and 4% (Controls). Causes of death was primarily AML, accounting for 73% and 88% of all deaths in the RICT and control groups, respectively. Twenty pts with an identified donor did not reach RICT due to relapse (n=12), co-morbidities (n=5), death (n=2), other (n=1).
Total mortality at time of analysis was slightly lower in the RICT group (66% vs 75%). Overall survival (primary endpoint) at 3 years was 45% (CI 33-56) and 48% (36-60), in RICT and control groups, respectively. At 10 years after inclusion, OS in study groups were similar; RICT 27% (CI 15-41), Control 25% (CI 15-36). There were no significant differences between study groups with respect to primary or secondary endpoints (OS: P=0.27, RFS:P=0.98, RI: P=0.50, NRM: P=0.10, logrank tests. Figure).
Conclusions. Applying an intention-to-treat analysis we did not demonstrate clinical benefit of sibling donor search and stem cell transplantation after a reduced intensity busulfan/fludarabine based regimen in AML patients ≥50 years in CR1. Early relapse was the main reason for preventing transplants in patients with an identified donor.
Support from study groups: Canadian BMT Group, Australasian Leukaemia and Lymphoma Group, Norwegian/Swedish BMT Group, Swedish AML group
Kiss:Alexion: Membership on an entity's Board of Directors or advisory committees, Research Funding; Otsuka: Membership on an entity's Board of Directors or advisory committees, Research Funding. Wallhult:Jazz Pharmaceuticals: Speakers Bureau; Amgen: Speakers Bureau; Daichii-Sankyo: Speakers Bureau. Finke:Riemser: Consultancy, Honoraria, Research Funding; Neovii: Consultancy, Honoraria, Other: travel grants, Research Funding; Novartis: Consultancy, Honoraria, Other: travel grants, Research Funding; Medac: Consultancy, Honoraria, Other: travel grants, Research Funding. Sabloff:Celgene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal